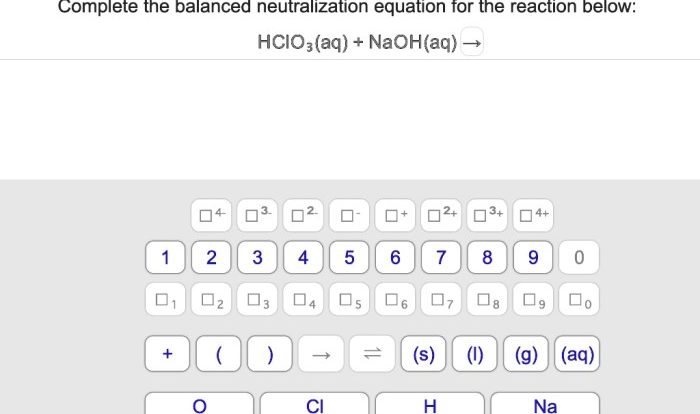
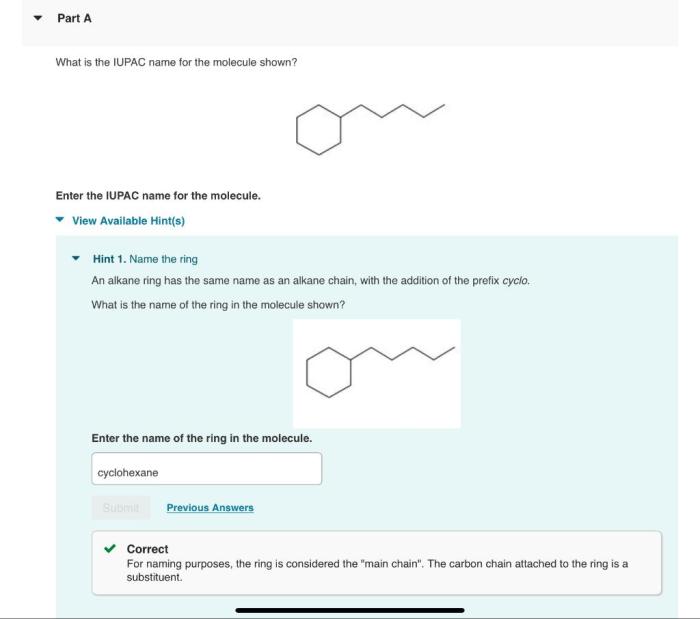
What is the iupac name for the molecule shown – Delving into the intricacies of IUPAC nomenclature, this exploration unravels the principles and methodologies employed to assign systematic names to organic molecules. Rooted in the guidelines established by the International Union of Pure and Applied Chemistry (IUPAC), this nomenclature system provides a standardized and globally recognized language for accurately describing the structure and composition of chemical compounds.
Through a comprehensive examination of functional group identification, parent chain determination, and substituent naming, this discourse elucidates the systematic approach to IUPAC nomenclature. By mastering these concepts, chemists gain the ability to decipher and construct IUPAC names, enabling effective communication and comprehension within the scientific community.
1. IUPAC Nomenclature Basics
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of guidelines for naming organic compounds. These guidelines help to ensure that organic compounds are named in a consistent and systematic manner.
The IUPAC nomenclature system is based on the following principles:
- The name of an organic compound is derived from its structure.
- The name of a parent chain is based on the number of carbon atoms in the chain.
- Functional groups are identified by their characteristic suffixes.
- Substituents are identified by their prefixes.
Examples of IUPAC Names for Simple Molecules, What is the iupac name for the molecule shown
- Methane: CH 4
- Ethane: C 2H 6
- Propane: C 3H 8
- Butane: C 4H 10
- Pentane: C 5H 12
2. Identifying Functional Groups
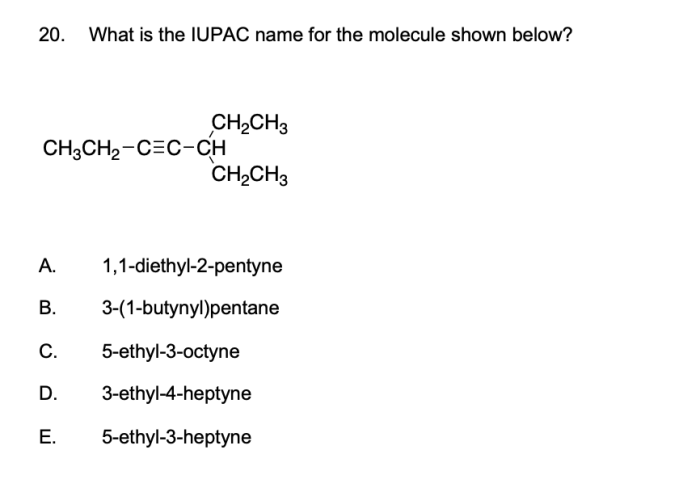
Functional groups are groups of atoms that have characteristic chemical properties. The most common functional groups include:
- Alkanes: Contain only carbon and hydrogen atoms and have the general formula C nH 2n+2.
- Alkenes: Contain a carbon-carbon double bond and have the general formula C nH 2n.
- Alkynes: Contain a carbon-carbon triple bond and have the general formula C nH 2n-2.
- Alcohols: Contain a hydroxyl group (-OH) and have the general formula R-OH.
- Ethers: Contain an ether group (-O-) and have the general formula R-O-R’.
- Aldehydes: Contain a carbonyl group (-C=O) and have the general formula R-CHO.
- Ketones: Contain a carbonyl group (-C=O) and have the general formula R-CO-R’.
- Carboxylic acids: Contain a carboxyl group (-COOH) and have the general formula R-COOH.
The priority of functional groups is determined by the following rules:
- Carboxylic acids have the highest priority.
- Aldehydes and ketones have the second highest priority.
- Alcohols, ethers, and amines have the third highest priority.
- Alkenes and alkynes have the fourth highest priority.
- Alkanes have the lowest priority.
3. Determining the Parent Chain
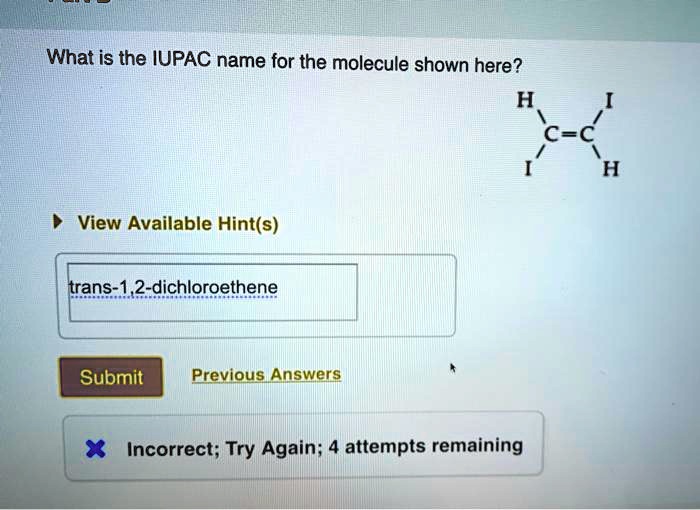
The parent chain is the longest continuous chain of carbon atoms in the molecule. The suffix of the parent chain is based on the number of carbon atoms in the chain.
- 1-3 carbon atoms: -ane
- 4 carbon atoms: -ene
- 5 carbon atoms: -yne
4. Numbering the Parent Chain: What Is The Iupac Name For The Molecule Shown
The parent chain is numbered so that the functional group with the highest priority has the lowest possible number.
The following rules are used to number the parent chain:
- Start numbering from the end of the chain that is closest to the functional group with the highest priority.
- If there is more than one functional group with the same priority, start numbering from the end of the chain that is closest to the functional group with the next highest priority.
- If there are multiple equivalent functional groups, start numbering from the end of the chain that gives the lowest possible numbers to all of the functional groups.
5. Naming Substituents
Substituents are groups of atoms that are attached to the parent chain.
The prefixes for substituents are based on the structure of the substituent.
- Alkyl groups: -yl
- Alkenyl groups: -enyl
- Alkynyl groups: -ynyl
- Halo groups: -o
- Hydroxy groups: -ol
- Ether groups: -oxy
- Aldehyde groups: -al
- Ketone groups: -one
- Carboxylic acid groups: -oic acid
6. Putting It All Together
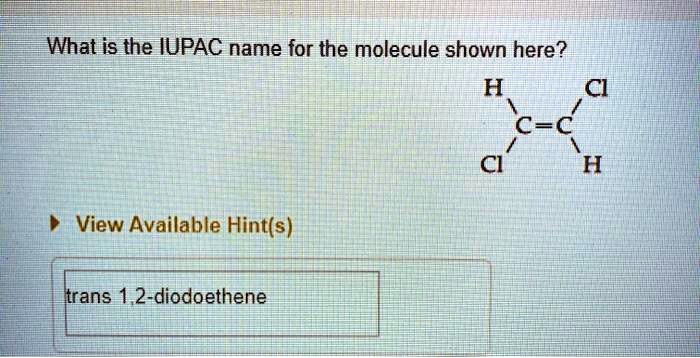
The IUPAC name of an organic compound is formed by combining the name of the parent chain, the names of the substituents, and the numbers that indicate the positions of the substituents on the parent chain.
The following steps are used to form the IUPAC name of an organic compound:
- Identify the parent chain.
- Number the parent chain.
- Identify the substituents.
- Name the substituents.
- Combine the name of the parent chain, the names of the substituents, and the numbers that indicate the positions of the substituents on the parent chain.
7. Examples of IUPAC Names
| Molecule | IUPAC Name |
|---|---|
| CH3CH2CH2CH3 | Butane |
| CH3CH2CH=CH2 | Butene |
| CH3CH2CH2CH2OH | Butanol |
| CH3CH2CH2CHO | Butanal |
| CH3CH2CH2COOH | Butanoic acid |
FAQ Compilation
What is the significance of IUPAC nomenclature?
IUPAC nomenclature provides a standardized and globally recognized system for naming organic molecules, ensuring clarity and consistency in chemical communication.
How is the parent chain determined in IUPAC nomenclature?
The parent chain is the longest continuous chain of carbon atoms in the molecule, and its name forms the основу of the IUPAC name.
What are functional groups and how do they influence IUPAC nomenclature?
Functional groups are specific arrangements of atoms or bonds that impart characteristic chemical properties to molecules. They determine the suffix of the IUPAC name and influence the priority of substituents.