Embarking on a journey through the periodic table crash course chemistry #4, we delve into the fascinating world of elements, their properties, and the intricate relationships that govern their behavior. This comprehensive guide unveils the secrets of the periodic table, providing a solid foundation for understanding the fundamental principles of chemistry.
Delving deeper into the periodic table’s organization, we explore the significance of atomic number, electron configuration, and chemical properties. The periodic table serves as a roadmap, guiding us through the vast landscape of elements, their similarities, and their differences.
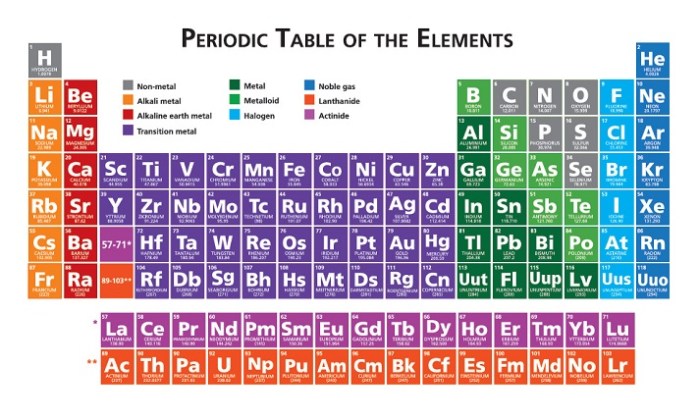
Introduction to the Periodic Table

The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. It is a powerful tool that helps us understand and predict the behavior of elements and their compounds.
Groups and Periods
The periodic table is divided into vertical columns called groups and horizontal rows called periods. Groups are numbered 1-18 from left to right, while periods are numbered 1-7 from top to bottom. Elements in the same group have the same number of valence electrons, which gives them similar chemical properties.
As we move across a period from left to right, the atomic number increases, and the elements become more electronegative and less reactive. As we move down a group, the atomic radius increases, and the elements become more metallic and reactive.
Blocks and Families
The periodic table is also divided into four blocks: s, p, d, and f. The s block contains the elements in groups 1 and 2, the p block contains the elements in groups 13-18, the d block contains the elements in groups 3-12, and the f block contains the lanthanides and actinides.
Elements in the same block have similar electron configurations, which gives them similar properties. For example, all s-block elements are highly reactive metals, all p-block elements are nonmetals or metalloids, and all d-block elements are transition metals.
Metals, Nonmetals, and Metalloids: The Periodic Table Crash Course Chemistry #4
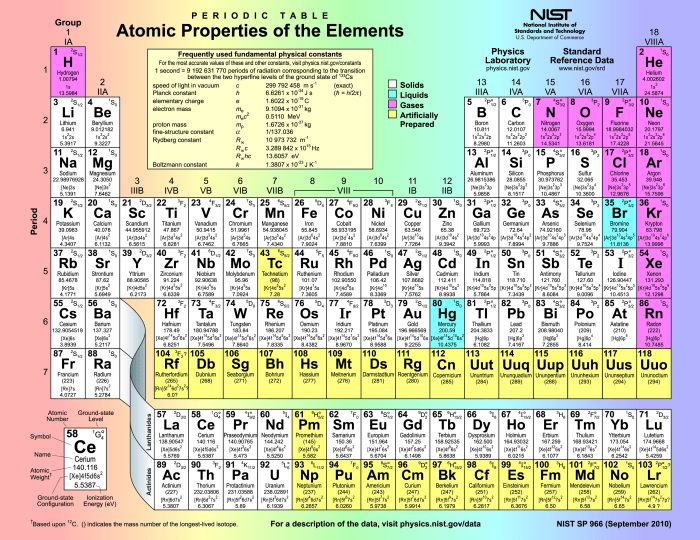
Metals are elements that are shiny, malleable, and ductile. They are good conductors of heat and electricity. Nonmetals are elements that are dull, brittle, and non-malleable. They are poor conductors of heat and electricity.
Metalloids are elements that have properties of both metals and nonmetals. They are typically shiny and malleable, but they are not as good conductors of heat and electricity as metals. Metals are located on the left side of the periodic table, nonmetals are located on the right side, and metalloids are located in between.
Trends and Periodic Properties
The periodic table shows several trends in atomic radius, ionization energy, electronegativity, and electron affinity. These trends can be used to predict the reactivity and behavior of elements.
Atomic radius decreases across a period from left to right and increases down a group. Ionization energy increases across a period from left to right and decreases down a group. Electronegativity increases across a period from left to right and decreases down a group.
Electron affinity increases across a period from left to right and increases down a group.
Applications of the Periodic Table
The periodic table is a powerful tool that is used in many fields of science, including chemistry, physics, and biology. It is used to design new materials, predict reactivity, and understand chemical processes.
The periodic table has also been used to develop new technologies, such as semiconductors and lasers. It is a fundamental tool that has helped us to understand the world around us and has played a major role in the development of modern science and technology.
General Inquiries
What is the purpose of the periodic table?
The periodic table organizes elements based on their atomic number, electron configuration, and chemical properties, providing a systematic framework for understanding and predicting their behavior.
How are elements organized in the periodic table?
Elements are arranged in the periodic table in rows (periods) and columns (groups), with elements sharing similar properties grouped together.
What are the different types of elements in the periodic table?
The periodic table categorizes elements as metals, nonmetals, and metalloids based on their properties, such as conductivity, malleability, and reactivity.