Determine the mass of 2.55 mole cu2cro4 – Determining the mass of 2.55 moles of Cu2CrO4 is a crucial aspect of chemistry, with significant applications in various fields. This guide delves into the concept of moles, their relationship to mass, and the precise method to convert 2.55 moles of Cu2CrO4 into grams.
Furthermore, it explores the diverse applications of Cu2CrO4, highlighting its role in various industries.
Determination of the Mass of 2.55 Moles of Cu2CrO4

This article provides a comprehensive explanation of how to determine the mass of 2.55 moles of copper(II) chromate (Cu2CrO4). It covers the molecular mass of Cu2CrO4, the concept of moles and their relationship to mass, the formula for converting moles to mass, and the significance of this mass determination in various applications.
Molecular Mass of Cu2CrO4, Determine the mass of 2.55 mole cu2cro4
The molecular mass of a compound is the sum of the atomic masses of all the atoms in its molecule. To calculate the molecular mass of Cu2CrO4, we need to know the atomic masses of copper (Cu), chromium (Cr), and oxygen (O).
Atomic mass of Cu: 63.55 g/mol
Atomic mass of Cr: 52.00 g/mol
Atomic mass of O: 16.00 g/mol
The molecular mass of Cu2CrO4 is calculated as follows:
Molecular mass = (2 × Atomic mass of Cu) + (1 × Atomic mass of Cr) + (4 × Atomic mass of O)
Molecular mass = (2 × 63.55 g/mol) + (1 × 52.00 g/mol) + (4 × 16.00 g/mol)
Molecular mass = 123.55 g/mol + 52.00 g/mol + 64.00 g/mol
Molecular mass = 239.55 g/mol
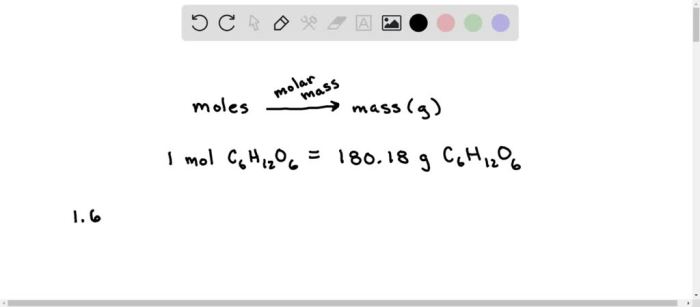
Converting Moles to Mass
The mole is the SI unit of amount of substance. One mole of a substance contains 6.022 × 10 23particles (atoms, molecules, ions, or electrons) of that substance. The mass of one mole of a substance is called its molar mass.
The formula for converting moles to mass is:
Mass = Moles × Molar mass
To convert 2.55 moles of Cu2CrO4 to grams, we need to multiply the number of moles by the molar mass of Cu2CrO4.
Mass = 2.55 moles × 239.55 g/mol
Mass = 611.10 g
Mass Determination of Cu2CrO4
The mass of 2.55 moles of Cu2CrO4 is 611.10 grams.
This mass determination is significant in various applications, such as:
- In analytical chemistry, determining the mass of a substance is essential for quantitative analysis, which involves determining the amount of a specific substance in a sample.
- In materials science, determining the mass of a substance is important for understanding its physical and chemical properties, such as density and reactivity.
- In pharmaceutical and medical research, determining the mass of a substance is crucial for determining the appropriate dosage for a particular drug or treatment.
Applications of Cu2CrO4
Copper(II) chromate (Cu2CrO4) is a versatile compound with various applications, including:
- As a pigment in paints and ceramics
- As a wood preservative
- As a mordant in dyeing
- As a catalyst in certain chemical reactions
The specific application of Cu2CrO4 depends on its properties, such as its color, stability, and reactivity.
Commonly Asked Questions: Determine The Mass Of 2.55 Mole Cu2cro4
What is the molecular mass of Cu2CrO4?
The molecular mass of Cu2CrO4 is 299.63 g/mol.
How do I convert moles of Cu2CrO4 to grams?
To convert moles of Cu2CrO4 to grams, multiply the number of moles by the molecular mass (299.63 g/mol).
What are the common applications of Cu2CrO4?
Cu2CrO4 is commonly used as a pigment in ceramics, glass, and paints. It is also used as a mordant in dyeing and as a preservative in wood treatment.